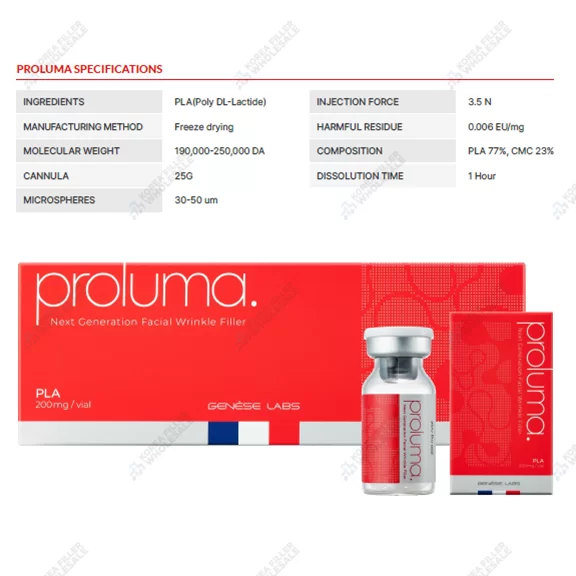
Description
Efficiency of Proluma
- New Tissues and Collagen Production:
- Week 2: Post-injection, cells begin adhering to the surface of PDLLA particles, filling interstitial spaces and promoting new tissue growth.
- Week 8: Cells completely occupy the spaces between PDLLA particles, migrating into their porous structure, as indicated by visual markers.
- Weeks 12 & 20: Cells deeply integrate within the PDLLA particles, significantly enhancing tissue regeneration and volumization.
- Long-Lasting Effect:
- Proluma not only corrects wrinkles and folds but also provides sustained volumization across facial areas. This durability stems from its ability to stimulate new collagen and tissue production, offering prolonged results compared to traditional fillers.
- Safety:
- PDLLA is known for its safety and non-toxic attributes, breaking down into harmless substances through natural body processes. Since 1984, it has been FDA approved and classified as Generally Recognized as Safe (GRAS).
How to Use Proluma:
- Preparation: Mix 7cc of Sterile Water For Injection (SWFI) and 2cc of lidocaine with the contents of the Proluma vial 30 minutes before the procedure.
- Mixing and Dissolution:
- Remove the vial cap, sanitize the stopper, and attach an 18G needle to a 7mL syringe. Draw 7mL of SWFI and inject it into the vial.
- Agitate vigorously for about 1 minute or until all components are dissolved.
- Before treatment, cleanse the rubber cap again, introduce 2cc of Lidocaine, and fill with the diluted solution using an 18G needle.
- Withdraw the suspension using a 1 or 3-mL sterile syringe.
- Replace the needle with a sterile 25G or 26G needle before injection.
- Injection Site: Administer into the deep dermis layer to effectively stimulate collagen production.
- Primary Target: Dry, thin skin with fine wrinkles.
- Secondary Target: Nasolabial folds.
Application Areas: Proluma is designed for deep dermis injection, actively enhancing collagen production more efficiently than conventional fillers.











Reviews
There are no reviews yet.